What are the procedures required for the certification of food display cabinets?
As refrigeration equipment that comes into direct or indirect contact with food, the certification process for food display cabinets must focus on two core aspects: safety performance (electrical and refrigeration) and food contact safety. Certification standards vary slightly across different countries and regions (e.g., China's 3C, EU's CE, US's UL).
Taking the mainstream domestic certification process as an example, a brief analysis is provided below:
1. Preliminary Preparation: Define standards and technical parameters
First determine the core standards the product must comply with: domestically, this requires meeting GB 4706.1 (General requirements for safety of household and similar electrical appliances) and GB 4706.32 (Particular requirements for safety of household and similar electrical appliances - Commercial refrigerated display cabinets). If food contact components are involved (such as the inner liner or shelves), compliance with the GB 4806 series (Safety Standard for Food Contact Materials and Articles).
Compile product technical documentation: including circuit diagrams, structural drawings, list of key components (e.g., compressor, motor, material certification for food contact materials), factory inspection reports, etc.
2. Submission for testing: Third-party laboratory assessment
Enterprises must submit representative samples (typically 1-2 units, identical to mass-produced products) to nationally accredited third-party testing bodies (e.g., China Household Electrical Appliances Research Institute, SGS).
Testing encompasses two categories:
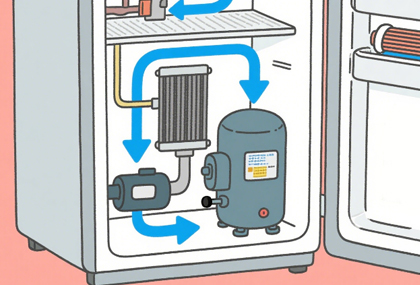
Safety Testing: Electrical safety (electrical shock protection, insulation resistance, earth resistance), refrigeration system safety (pressure testing, leak detection), temperature rise testing (preventing component overheating), mechanical strength (tip-over prevention, glass impact resistance), etc.;
Food contact testing: Migration testing for components in contact with food (e.g., heavy metals, hazardous substance migration), sensory testing (absence of odour, discolouration), etc.
Upon passing inspection, the laboratory issues a test report; failing products require modification according to rectification recommendations before resubmission.
3. Certification Application and Audit
For mandatory domestic certifications (e.g., 3C certification for certain commercial food display cabinets), enterprises must submit applications to certification bodies authorised by the China National Certification and Accreditation Administration (CNCA), providing materials such as business licences, technical documentation, and test reports.
The certification body reviews the materials: verifying document completeness and test report validity. If materials meet requirements, the process proceeds to the next stage; if queries arise, the enterprise must provide supplementary explanations or additional evidence.
4. Factory Audit (Required for Certain Certifications)
For certifications such as 3C or CE (European Union), where products fall under the ‘factory audit required’ category, the certification body will dispatch auditors to the enterprise's production site to verify:
Production Consistency: Ensuring mass production processes align with the technical specifications and component sourcing of submitted test samples;
Quality Control System: Verification of established protocols and documentation for raw material inspection, in-process monitoring, and final product testing;
Key Component Management: Confirmation of compliant, standard-conforming components (e.g., compressors must possess certification).
5. Certification Issuance and Ongoing Maintenance
Upon successful completion of material audits and factory inspections (where applicable), the certification body issues the relevant certificate (e.g., 3C certificate, CE certificate), typically valid for five years.
Ongoing Maintenance: Manufacturers must maintain production consistency. Certification bodies conduct periodic ‘surveillance audits’ (e.g., annually). Any alterations to product design or critical components require prior submission of a ‘change notification’ to the certification body to prevent certificate invalidation.
In essence, the core process involves ‘standard-based preparation → third-party testing → certification body review → post-certification maintenance’, ensuring products meet market access requirements for safety and food contact compliance.
Most popular More «
-

Ideal temperature and temperature control points of cake cabinet
-
Which Cooluma compact commercial cake display cabinet is the best?
-
What are the procedures required for the certification of food display cabinets?
-
Aims to be a global supplier of commercial cold chain equipment, Cooluma
-
Best Automatic Refrigerated Display Cabinet Series
-
Is Cooluma a Professional Cold Chain Equipment Service Provider?
-
Where Lies the Value of the Best Cake Display Showcase?
-
What are the advantages of a room-temperature cake display cabinet?
-
How much does a 120L bread cabinet cost?
-
Functions and Maintenance Details of Coffee Display Cabinets